William Lubell
University of Montreal
Talk Session: SESSION 11: SCAFFOLDS AND PEPTIDOMIMETICS
Date: Wednesday, June 15, 2022
Talk Time: 11:00 am - 11:20 am
Talk Title: Peptide Mimicry Using N-Aminoimidazole-2-ones
Professor at the Université de Montréal, I have been actively investigating solution-phase and solid-phase synthesis of heterocycles, amino acids, peptides, peptide mimics and the preparation of their libraries by combinatorial science. My interests lie both in developing new methodology for effectively synthesizing these novel structures for drug discovery, as well as in their use to explore protein folding, molecular recognition and bioorganic catalysis.
Since 2000, this research has received and we gratefully acknowledge support from the National Science and Engineering Research Council of Canada, the Medical Research Council of Canada, the Ministry of Education of Québec, Novo Nordisk Denmark, Merck Frosst Inc., ConjuChem Inc., Gemin-X Inc., BioAxone Inc. and Pharma-G Inc. Montréal, Valorisation-Recherche Québec and the Canadian Foundation for Innovation.
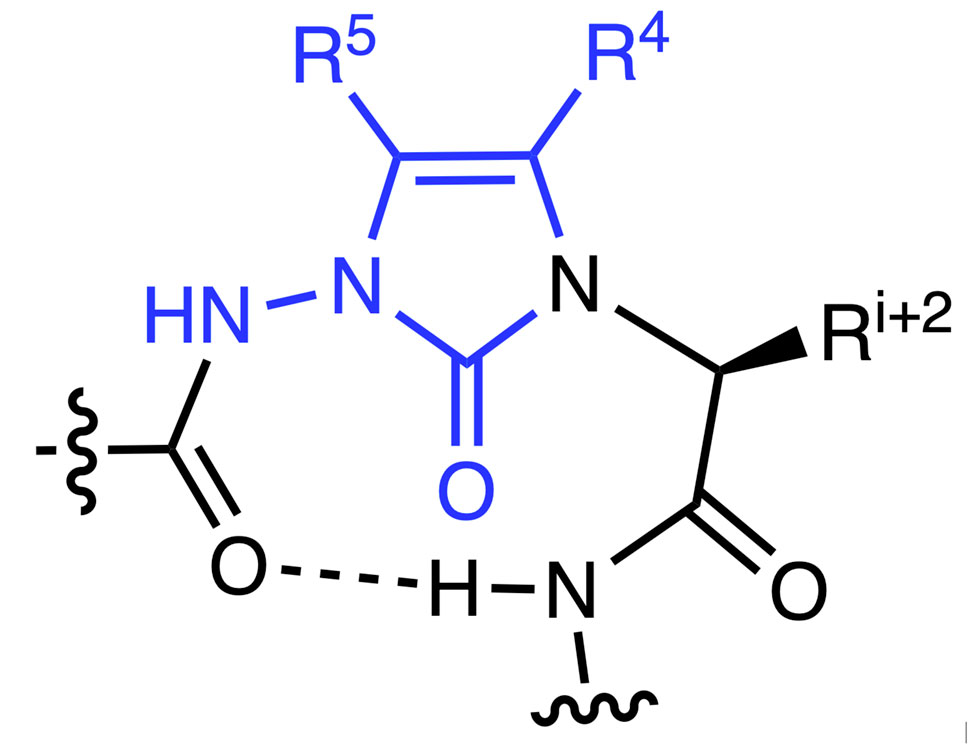
α-Amino lactam residues have been historically used to constrain peptide backbone conformation for studying structure-activity relationships in peptide-based drug discovery. N-Aminoimidazol-2-one, Nai, residues are aza-α-amino lactam counterparts. Synthesized by a route featuring proline-catalyzed alkylation of azopeptides, Nai residues can adopt the central position of β- and γ-turn secondary structures as illustrated by X-ray crystallographic and computational methods. Moreover, substituents can be readily added onto the Nai heterocycle for mimicry of different side chain orientations.
N-Aminoimidazol-2-one, Nai, residue in β-turn
With focus on structure-activity relationship studies of biologically active peptides, the synthesis, and applications of Nai residues will be presented.